The story so far
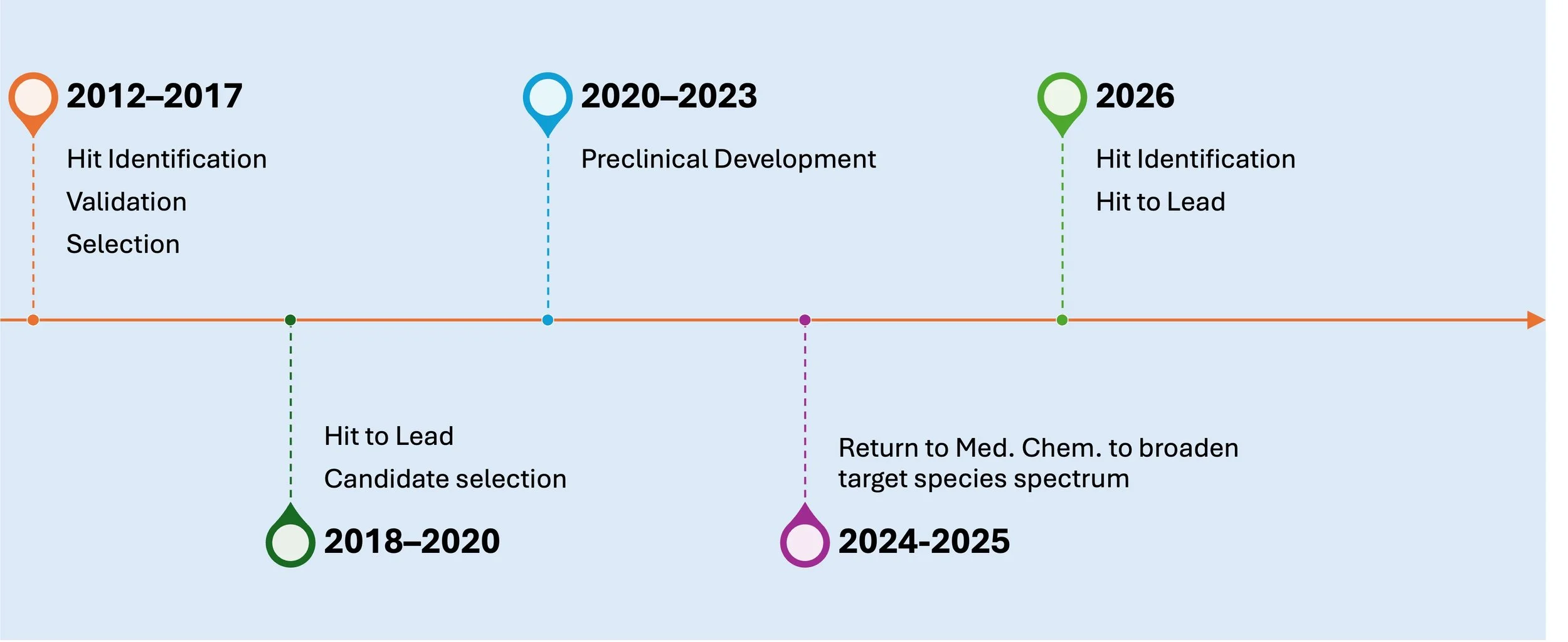
Development pathway for Disperazol as a New Chemical Entity for the treatment of Catheter Associated UTIs
The preclinical developmental status of Disperazol
Disperazol® Version 1 has patent pending status (PCT/EP2021/073753).
Urinary tract infections, cystic fibrosis-associated respiratory infections and recalcitrant wound infections have been identified as feasible and appropriate indications.
Development to date has shown repeatable evidence that Disperazol® clears catheter associated urinary tract infections in vivo with a therapeutically feasible dose using multiple routes of administration.
No signs of toxicity have been shown in testing to date.
The next steps for Disperazol Pharma are the development of second generation compounds that affect Escherichia coli and Klebsiella pneumoniae and not only Pseudomonas aeruginosa.
2010
In 2010 Prof. Michael Givskov and Prof. Tim Tolker-Nielsen merged their scientific teams to embark on a new intervention strategy that would deal with drug resistant bacterial infections. They envisioned a “magic bullet” in the form of a small, chemical entity that would activate the process of dispersing bacteria from a biofilm, making the bacteria susceptible to efficient killing by standard of care antibiotics.
Prof Givskov and Prof Tolker-Nielsen focused on a putative new antimicrobial target; the c-di-GMP signaling system, which controls the biofilm life cycle in Gram negative bacteria. It is now well-accepted that c-di-GMP serves as the central biofilm (sessile) – planktonic (free-living) decision maker in Gram-negative bacteria. As such, the c-di-GMP signaling system is a “non-lethal” drug target that is fundamentally different from the classic antibiotic targets and modes of action. c-di-GMP is not produced by humans suggesting that c-di-GMP signaling could be a biofilm-specific drug target with reduced risk of off-target effects.
2013-2014
In 2013 Assoc Prof. Jens Bo Andersen and Dr. Louise Hultqvist delivered an early “Proof of Concept” by plummeting bacterial c-di-GMP levels with a genetic construct, in which a PDE (c-di-GMP degrading enzyme) could be over-expressed by addition of arabinose. This was found to subsequently dismantle P. aeruginosa biofilms and eradicate P. aeruginosa biofilm infections in mice.
2015
One of the molecules identified in the screening campaign, now designated “H6”, appeared particularly promising because it fulfilled our expectations with respect to mode of action. Assoc. Prof. Katrine Qvortrup and her team at DTU-Chemistry joined the project in 2015, with the aim of optimizing the H6 hit by a structure-activity relationship analysis. Two of her undergraduates at that time, particularly PhD student Charlotte Uldahl Jansen and MSc student Jesper Uhd, de novo synthesised 70+ structural variants of the original H6 scaffold. Jesper Uhd then developed a water-soluble hydrochloride salt of H6-P1. This salt reduces c-di-GMP signaling, prevents biofilm formation and dismantles already established biofilms in a concentration dependent manner. The H6-P1 salt is currently the drug candidate behind the name Disperazol®.
2017-2020
In 2016, Dr Michael Graz joined the team. His role is commercial development and funding and ensuring that the research is performed in a manner to allow for clinical development. He holds a track record of mentoring biopharmaceutical start-ups, and leading and turning around both scale-up and mature life sciences companies in the UK, South Africa and South America.
From 2017 Assoc Profs. Louise Hultqvist and Carl Martin Nilsson delivered experimental proof that the H6-P1 mode of action worked well in concert with approved antibiotics to kill biofilm bacteria and eradicate a biofilm infection. During 2019 and 2020, Jens Bo Andersen, Louise Hultqvist and Carl Martin Nilsson found the likely drug target to be the phosphodiestrase BifA, that affects c-di-GMP concentrations in P. aeruginosa.
2021
Prof Claus Moser, Chief physician at KMA Rigshospitalet joined the team in 2021. Prof Moser has more than 20 years of experience with biofilm infections. His extensive clinical background, clinical collaborators, and previous experience from clinical trials, makes him suitable to lead clinical development of the new anti-biofilm drug candidate Disperazol and in vivo treatment scenarios that include Disperazol combination with antibiotics.
2022-2025
The team developed a model for P.aeruginosa biofilms on catheters and demonstrated biofilm dismantling efficacy of Disperazol within an hour after oral administration. Furthermore, we have shown that Disperazol works synergistically with approved antibiotics to eradicate a biofilm infection. All results suggest that Disperazol and closest variants are safe and can be considered as drug-able independent of administration route.
The Disperazol team now has access to complementary competencies of experts in microbiology and molecular biology, chemical synthesis, medicine and pharmaceutical technology and commercial development. These competencies will be supported by expertise in patient care and patient advocacy to ensure that the project outcome will be a therapeutic that will be used by clinicians and accepted by patients.
2026
In January 2026, Disperazol partenered with Biophys Limited in Cardiff, Wales (UK), to support its development of a second generation asset based on compounds developed on our behalf by CC4CARB in the USA.